From August 14th to 16th, the WORLD OF CONCRETE ASIA 2024 (WOCA) will be held at the Shanghai New International Exposition Facility, China. Luoyang Tongrun Details Technology Co., Ltd will certainly participate in the exhibition( Booth Number: E1C01). The product will certainly cover a selection of applications, such as concrete frothing agents, polycarboxylate superplasticizers, and instantaneous salt silicate powder, in order to explore even more business opportunities with brand-new and old clients.
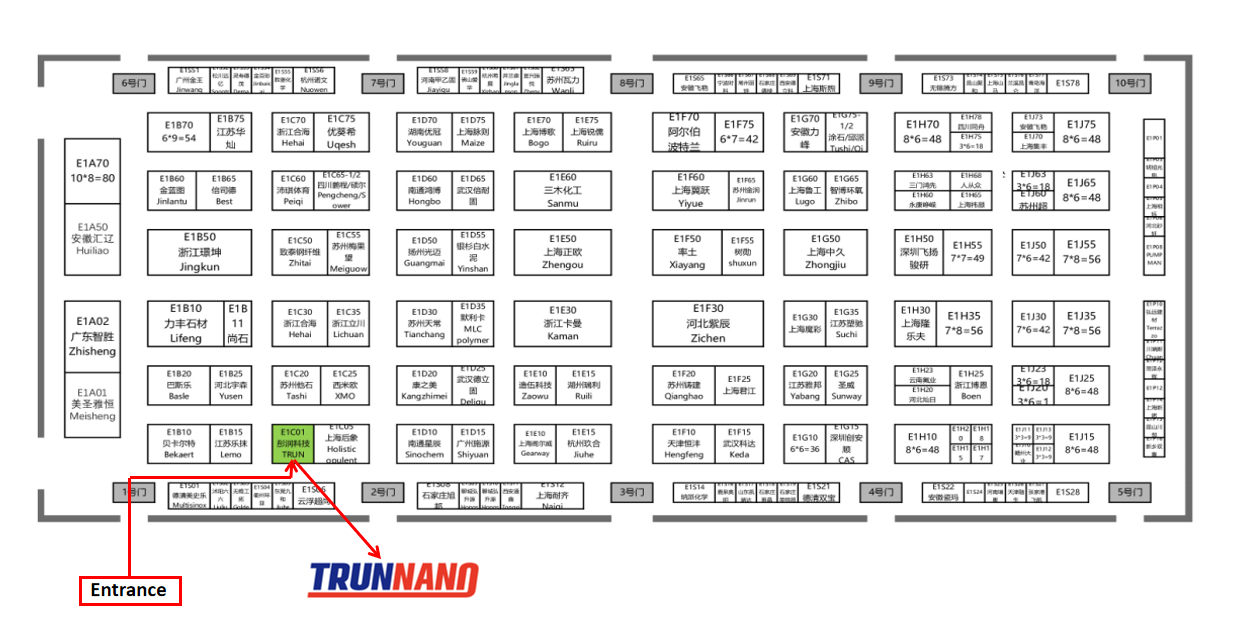
(TRUNNANO Booth Number: E1C01)
1 . Firm Fundamental Information
Luoyang Tongrun Info Innovation Co., Ltd is one of the exhibitors of this event. It is a thorough industrial firm incorporating R&D, production, and sales. The primary items are cement frothing representatives, polycarboxylate superplasticizers, water-based mould-releasing agents, self-insulating block admixtures, and lightweight wall surface panel admixtures. TRUNNANO Modern technology is the source supplier of ingredients for the environment-friendly structure energy-saving market.
Luoyang Tongrun Nano Modern Technology Co., Ltd. has 35 staff members. Through the constant advancement of brand-new innovations and products, it has actually effectively gotten 9 licenses. In 2018, it was called a “modern venture.”

( TRUNNANO(Luoyang Tongrun) Logo)
2 . Company Standard Information
Product A: Concrete foaming agent
Concrete frothing representative is an additive that can minimize the surface stress of liquid, produce a multitude of uniform and steady foams, and be utilized to create foamed concrete. TRUNNANO lightweight concrete team has actually launched 4 series of high-performance lathering representatives (TR-A, TR-B, TRC, TR-D) that, integrated with 14 years of experience in the sector, according to the different demands of the market, can meet various building requirements.
Concrete frothing representative is extensively used in light-weight partition boards, CLC blocks, backfill, etc

( TRUNNANO Concrete Foaming Agent)
Item B: Polycarboxylate Superplasticizer
Superplasticizer is a concrete admixture that can minimize the water consumption of blending under the condition of preserving the slump of concrete unmodified. Superplasticizer has a dispersing result on concrete fragments, which can improve its working performance, lower the water usage per unit, improve the fluidness of concrete mix, or reduce the quantity of cement each, saving cement. TRUNNANO superplasticizer is changed on its original process so that it can be well applied in lathered cement without defoaming.

( TRUNNANO Polycarboxylate Superplasticizer)
Polycarboxylate Superplasticizer is a cement dispersant made use of in cement concrete. It is commonly utilized in highways, bridges, dams, passages, high-rise buildings and other jobs.
Product C: Instantaneous Salt Silicate Powder
Immediate sodium silicate is a white fine-grained product that can be dissolved in water quickly. It is an unique type of bubbly antacids with the qualities of chilly resistance, homogeneity, and exceptionally hassle-free usage, transportation, and storage. Instantaneous salt silicate is mainly made use of in refractory sintering agents, cleaning complementary representatives, dirt conditioners, ore dressing preventions, acid-resistant concrete additives, chemical grouting auxiliary agents, water therapy, and other areas.

( TRUNNANO Instant Sodium Silicate Powder)
Instant salt silicate is mostly used as a cleaning agent aid in artificial detergents, a quick-drying and enhancing agent for concrete, a drill cuttings deposition agent for silicon-based boring fluids, and an anti-expansion agent for mud shale
Product D: Fluid Lithium Silicate
Liquid lithium silicate is an anemic and transparent water-soluble substance with exceptional solubility and can be swiftly and uniformly dispersed in water. Liquid lithium silicate has great cold resistance and stability.

( TRUNNANO Liquid Lithium Silicate)
Liquid lithium silicate can be made use of to prepare special glasses and porcelains, which can boost the heat resistance and mechanical toughness of items; liquid lithium silicate can be made use of as a basic material for advanced corrosion-resistant finishings and sealing materials in the structure materials market; fluid silicic acid As one of the electrolyte parts, lithium is used in the battery manufacturing sector to assist boost the power density and cycle stability of lithium-ion batteries; as a catalyst or catalyst provider, it promotes the effective conduct of certain chain reaction.
Item E: Immediate Potassium Silicate Powder & Fluid Potassium Silicate
Instantaneous potassium silicate powder and fluid potassium silicate are highly efficient and multi-functional chemical products with superb water solubility and large range of industrial applications. The powder form is white, soluble and simple to lug, supplying a stable solution for refractory products, metal anti-corrosion and farming improvement; while the liquid type reveals greater versatility and environmental management in the areas of concrete strengthening, water therapy and steel handling.

( TRUNNANO Instant Potassium Silicate Powder)
Potassium silicate is commonly made use of in welding rod manufacturing, welding electrodes, glass industry to improve product efficiency, anti-corrosion coverings, casting and agricultural soil enhancement to enhance crop resistance and high quality.
For more information, please visit: www.nanotrun.com (tech@nanotrun.com) .
Inquiry us